How citrullination of CAMP contributes to IBD: A new mechanism of immune dysfunction
FAYETTEVILLE, GA, UNITED STATES, December 6, 2025 /EINPresswire.com/ -- Cathelicidin (CAMP) is a critical antimicrobial peptide known for its protective roles in immune regulation, pathogen defense, and epithelial barrier maintenance. New research investigates how citrullination of CAMP, mediated by peptidyl arginine deiminase 4 (PAD4), exacerbates inflammatory bowel disease (IBD).
Inflammatory bowel disease (IBD), encompassing conditions such as ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic inflammation of the gastrointestinal tract. Despite advances in treatment, the mechanisms underlying IBD remain complex and poorly understood. Emerging research highlights the role of antimicrobial peptides, like cathelicidin (CAMP), in modulating gut inflammation and microbiota balance. However, the post-translational modifications of CAMP, particularly citrullination, and their impact on IBD have not been fully explored. Based on these challenges, further investigation is needed to elucidate the precise role of CAMP modification in IBD and to identify potential therapeutic strategies.
Researchers from Changhai Hospital and Naval Medical University, published (DOI: 10.1093/pcmedi/pbaf023) in Precision Clinical Medicine, examine how citrullination of the antimicrobial peptide CAMP exacerbates inflammation in IBD. The study reveals that PAD4-mediated citrullination of CAMP alters microbiota composition and immune cell responses, highlighting a novel pathway in IBD pathogenesis.
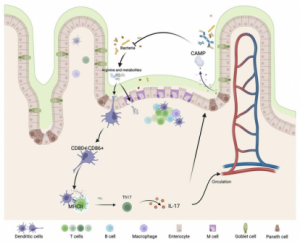
Through a dextran sodium sulfate (DSS)-induced colitis mouse model, researchers found that citrullination of CAMP, driven by PAD4, significantly reduced its protein levels in the intestines. This modification did not affect CAMP transcription but led to its degradation and diminished protective functions. Metaproteomic analyses revealed 70 differentially expressed proteins and 15 altered microbiota families associated with CAMP deficiency. The absence of CAMP intensified intestinal inflammation, as evidenced by increased pro-inflammatory cytokine levels and disrupted epithelial barrier function. Furthermore, the research demonstrated that the citrullinated form of CAMP contributed to an imbalance in microbial communities, particularly affecting arginine metabolism and promoting dendritic cell (DC) maturation and Th17 polarization. These findings underscore the dual role of CAMP in both microbiota modulation and immune response regulation during IBD.
"Understanding the post-translational modifications of antimicrobial peptide like CAMP provides critical insights into the complexities of IBD," says Dr. Zhaoshen Li, one of the corresponding authors. "By targeting PAD4-mediated citrullination, we may open new therapeutic avenues for improving the treatment and management of IBD."
These findings have significant implications for IBD therapy. Modulating CAMP levels through PAD4 inhibition or preventing citrullination could potentially restore intestinal barrier function and normalize microbiota composition. Future research should focus on validating these results in human models and exploring the therapeutic potential of CAMP-based treatments or microbiota-modulating strategies in IBD.
References
DOI
10.1093/pcmedi/pbaf023
Original Source URL
https://doi.org/10.1093/pcmedi/pbaf023
Funding Information
This study was supported by the National Natural Science Foundation of China (grant Nos. 82100587 and 82400605), the China National Postdoctoral Program for Innovative Talents (grant No. BX20220288), the China Postdoctoral Science Foundation (grant No. 2022M720138), the Clinical Research Special Project of Shanghai Municipal Health Commission (grant No. 20244Y0209), Capability Enhancement Project for Clinical Research Physician of the First Affiliated Hospital of Naval Medical University (grant No. 2024LYC01), the Basic Medical Research Project of the First Affiliated Hospital of Naval Medical University (grant No. 2023PY06), the Basic Medical Research Projects of Naval Medical University (grant Nos. 2024MS08 and 2024QN026), the “Yuanhang” Talent Program of Naval Medical University, the “Changjian” Talent Program of Changhai Hospital of Naval Medical University, the “Changying” Talent Program of Changhai Hospital of Naval Medical University and Youth Project of Shanghai Key Laboratory (grant Nos. 2025QN01 and 2025QN09).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.